Introduction:
Paricalcitol is a synthetic form of vitamin D used to manage and prevent secondary hyperparathyroidism associated with long-standing kidney failure. Paricalcitol is an analog of 1,25-dihydroxy ergocalciferol, an active form of vitamin D2 or ergocalciferol. The drug regulates the levels of natural substances made by the parathyroid gland. The drug is effective for patients with elevated parathyroid levels and low calcium and vitamin D levels. An excess parathyroid hormone can cause bone disorders. Hence, Paricalcitol, a synthetic form of vitamin D, can reduce parathyroid levels, thereby increasing the absorption of phosphorus and calcium. Paricalcitol is currently manufactured in the US (United States) for Abbott by Tetrionics. The drug is synthesized using 27 steps, and the final product obtained has 99.8 % purity. Finally, Paricalcitol capsules were approved by the Food and Drug Administration on 26th May 2005. In contrast, the injection was approved on 17th April 1998.
Safety and Effectiveness of Paricalcitol:
Paricalcitol proved to be effective in treating secondary hyperparathyroidism and lowering phosphorus and calcium levels. The drug has been approved in more than 60 countries to prevent hyperparathyroidism due to chronic kidney failure. Clinical studies reveal that Paricalcitol can prolong the survival time in patients undergoing dialysis for long periods. In addition, the drug can lower parathyroid levels in patients with elevated phosphorus levels. However, some studies found that the drug does not provide any additional benefits and causes side effects similar to those observed with Alfacalcidol.
How Does Paricalcitol Work to Treat Secondary Hyperparathyroidism?
Patients with chronic renal failure often present with secondary hyperparathyroidism due to depressed calcium and phosphate levels. This is an important factor in the pathogenesis of secondary hyperparathyroidism. Vitamin D analogs like Paricalcitol enhance the absorption of calcium and phosphorus from the intestines. In addition, the drug stimulates the reabsorption of Paricalcitol from the kidneys. As a result, the patient has elevated calcium and phosphorus levels. Hence, improvements in bone strength are commonly observed in people taking Paricalcitol.
For Patients:
Secondary Hyperparathyroidism:
Secondary hyperparathyroidism is a condition wherein the parathyroid glands release excess parathyroid hormone. In addition, the glands increase in size resulting in elevated parathyroid levels in the blood. Patients with kidney disease tend to experience secondary hyperparathyroidism due to elevated phosphorus levels, vitamin D deficiency, and decreased blood calcium levels. Secondary hyperparathyroidism can initiate bone disease and the build-up of calcium in the tissues and vital organs like blood vessels and the heart.
Symptoms of Secondary Hyperparathyroidism:
Secondary hyperparathyroidism occurs in response to various other renal conditions, including end-stage renal disease. The signs and symptoms of secondary hyperparathyroidism are listed below:
-
Fractures.
-
Bone deformities.
-
Calcium deposition in the tissues.
-
Itching of the skin.
-
Non-healing wounds.
What Is Paricalcitol?
Paricalcitol is simply a synthetic form of active vitamin D. Active vitamin D plays a crucial role in regulating the functioning of different body tissues, including the bones and the parathyroid gland. For people with normal kidney function, the active form of vitamin D is synthesized within the body. However, in patients with kidney failure, the production of the active form of vitamin D is significantly reduced. Hence, Paricalcitol is an important source of vitamin D for patients whose bodies fail to produce enough vitamins. In addition, the drug saves one from the consequences of elevated parathyroid levels, which can initiate bone problems. Therefore, Paricalcitol is effectively used in patients with stage 3, 4, or 5 kidney disease and children between 10 to 16 years of age.
What Should the Patient Inform the Doctor Before Taking Paricalcitol?
Before taking Paricalcitol, one must inform the doctor if one is:
-
Is allergic to Paricalcitol or any of its constituents.
-
Has elevated vitamin D or calcium levels.
-
Has been diagnosed with the following conditions:
-
Heart disease.
-
Irregular heartbeat.
-
Coronary heart disease.
-
Seizures.
-
-
Is planning to undergo any dental surgery.
-
Is taking or has consumed prescription drugs, herbal, vitamin, or mineral supplements.
-
Is pregnant or planning on the same.
-
Is currently breastfeeding.
Warnings and Precautions:
-
Before initiating Paricalcitol therapy, the patient needs to limit phosphorus consumption in the diet.
-
The patient can start taking phosphate-binding medicines to keep a check on the phosphorus levels.
-
The doctor might adjust the dose for patients taking calcium-based phosphate binders.
-
Sometimes, the doctor might recommend a blood test to monitor the patient's treatment.
-
Patients with stage 3 and 4 chronic kidney disease often present with increased blood creatinine levels. However, it does not affect a patient’s renal functions.
How Should the Patient Take Paricalcitol?
The patient must take Paricalcitol exactly as recommended by the doctor. Avoid taking an excess dose for a prolonged period unless mentioned by the doctor. Paricalcitol can be taken with or without food. However, the patient must follow the doctor’s instructions related to the diet. Without the doctor's consent, the patient must not consume any vitamin D, calcium, phosphate supplements, or antacids containing aluminum. Before initiating the therapy, the doctor might ask the patient to limit the consumption of phosphate-rich foods like beer, chocolate, cola, cheese, ice creams, peas, nuts, or milk. In addition, patients must not consume Paricalcitol with cholestyramine or mineral oil. Instead, the drug must be taken an hour before or four to six hours after taking cholestyramine. Avoid eating grapefruit while taking Paricalcitol.
How Should the Patient Store Paricalcitol?
Paricalcitol must be kept away from the reach of children. The drug need not be stored under any special conditions. Avoid using the drug after the expiry date stated on the carton or label. Avoid discarding the medicine in any household waste. The drug must be disposed of according to community guidelines to protect the environment.
What Are the Side Effects of Paricalcitol?
Some of the serious side effects of Paricalcitol are listed below:
-
Shortness of breath.
-
Wheezing.
-
Rashes.
-
Itching.
-
Swelling of the lips and face.
Common side effects affecting one out of ten individuals include increased blood calcium and phosphate levels. In addition, the patient might experience the following uncommon side effects:
-
Pneumonia.
-
Reduced parathyroid levels.
-
Dizziness.
-
Unusual taste in the mouth.
-
Decreased calcium levels.
-
Headache.
-
Irregular heartbeat.
-
Abdominal discomfort.
-
Constipation.
-
Diarrhea.
-
Dryness of mouth.
-
Nausea.
-
Vomiting.
-
Itchiness of the skin.
-
Acne.
-
Hives.
-
Rashes.
-
Muscle cramps.
-
Weakness.
-
Tiredness.
-
Swollen legs.
-
Altered liver function tests.
For Doctors - Paricalcitol Injection and Capsules:
Indications and Usage:
Paricalcitol is indicated for adults and children ten years of age and above to treat and prevent secondary hyperparathyroidism associated with stage 3 or 4 chronic kidney disease. In addition, the drug can also be given to patients with secondary hyperparathyroidism associated with stage 5 chronic kidney disease who are on hemodialysis or peritoneal dialysis.
Dosage and Administration - Paricalcitol Capsules:
Stage 3 and 4 Chronic Kidney Disease:
Paricalcitol capsules must be given once daily or thrice a week. However, one must not take the drug more than three times a week.
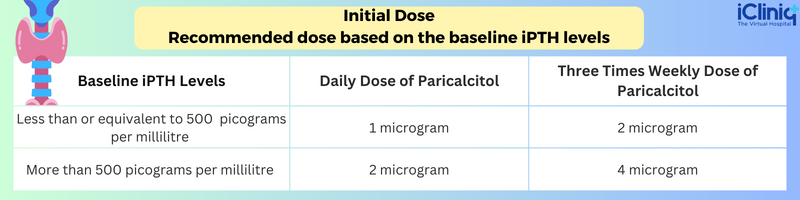
Initial Dose:
Recommended dose based on the baseline iPTH levels:
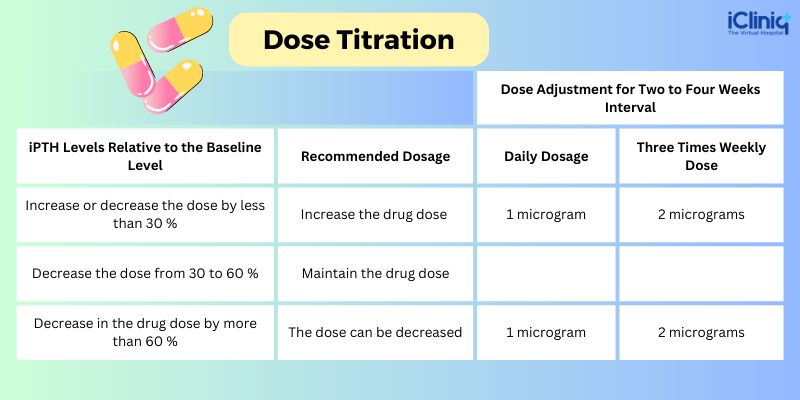
Dose Titration:
Pediatric Patients 10 to 16 Years of Age:
Chronic Kidney Disease Stages 3 and 4:
Initial Dose:
Paricalcitol 1 mg capsule can be given three times a week in children.
Dose Titration:
Paricalcitol dosage can be titrated based on serum calcium, phosphorus, and iPTH levels. The drug dose might be increased by 1 mcg every four weeks. However, the doctor might decrease the administered dose by 1 mcg. The patient might be asked to discontinue the drug if the dose reduction is required beyond 1 mcg.
Monitoring the Therapy:
Serum calcium and phosphorus levels of patients taking Paricalcitol must be carefully evaluated during dose titration and concomitant administration with strong CYP3A inhibitors. If patients present with hypocalcemia, they might be asked to reduce or withhold the drug dose.
Dosage Forms and Strength:
Paricalcitol capsules are available as follows:
-
1 mcg - They are oval and gray colored with “a” embossed on one side and the brand name on the other side.
-
2 mcg - They are orange-brown capsules with “a” embossed on one side and the brand name on the other side.
Contraindications:
Paricalcitol must be avoided in patients with hypercalcemia and vitamin D toxicity.
Dosage and Administration - Paricalcitol Injection:
Important Administration Instructions:
-
The doctor must ensure that the patient’s serum calcium levels are in the normal range.
-
The drug can be administered intravenously using a hemodialysis vascular access port at any time of dialysis.
-
The doctor must carefully inspect the Paricalcitol solution before administration. The solution should be used only if it appears clear or colorless.
-
Discard the unused portions of solution in 2 mg per mL and 5 mg per mL single-dose vials.
Initial Dose and Titration Dose in Adults:
-
The drug must be administered as an intravenous bolus at the dose of 0.04 mcg per kg to 0.1 mcg per kg every other day at the time of dialysis.
-
Paricalcitol dose must be adjusted to target the parathyroid hormone levels and maintain the serum calcium levels in the required range.
-
The patient’s serum calcium and parathyroid hormone levels must be evaluated every two to four weeks.
-
The drug dose can be titrated based on intact parathyroid (PTH) levels. The maximum daily dose for adults is 0.24 mcg per kg.
-
Paricalcitol therapy can be suspended or withheld if PTH levels are abnormally low to sustain bone health. In addition, the patient must restart the dose only after the laboratory values have normalized.
Dosage Forms and Strengths:
Paricalcitol solution is clear, colorless, and available as follows:
-
2 mcg per mL single-dose vial.
-
5 mcg per mL single-dose vial.
-
10 mcg per 2 mL multiple dose vial.
Warnings and Precautions:
-
Hypercalcemia - Hypercalcemia or elevated calcium levels is one of the most significant problems associated with Paricalcitol. Sometimes, the condition becomes so severe that it requires emergency medical attention. The patient might develop cardiac arrhythmias and seizures, which might potentiate the action of Digitalis. Chronic hypercalcemia can cause generalized vascular and soft tissue calcifications. In addition, the coadministration of Thiazide diuretics and calcium-containing drugs can increase the risk of serum abnormalities. As a result, the patient needs to be monitored frequently and requires frequent dose titrations. The doctor must inform the patient about the following symptoms of elevated calcium levels:
-
Tiredness.
-
Impaired thinking abilities.
-
Nausea.
-
Loss of appetite.
-
Vomiting.
-
Constipation.
-
Increased thirst.
-
Increased frequency of passing urine.
-
Weight loss.
-
-
Digitalis Toxicity - Hypercalcemia can exacerbate Digitalis toxicity. Hence, Paricalcitol must be used cautiously in patients taking Digitalis.
-
Bone Diseases - The patient might acquire adynamic bone diseases if Paricalcitol excessively suppresses PTH levels.
-
Aluminum Toxicity - Aluminium continuing antacids or phosphate binders must not be administered along with Paricalcitol to avoid the risk of aluminum toxicity in bones.
Description:
Paricalcitol is a synthetically manufactured analog of active vitamin D with modifications in the side chain (D2) and the A (19-nor) ring. Paricalcitol capsules are white and made of soft gelatin meant for oral administration. Each capsule comprises medium-chain triglycerides, butylated hydroxytoluene, and alcohol. The medium-chain triglycerides are manufactured from coconut oil or palm kernel oil. The empirical or molecular formula of the drug is C27H44O3, whereas the molecular weight is 416.64.
Clinical Pharmacology:
Mechanism of Action:
Paricalcitol is a biologically active vitamin D2 analog of calcitriol. In vitro, and preclinical studies reveal that the biological actions of the drug are mediated through the binding of vitamin D receptors. As a result, the vitamin D pathways get activated. Paricalcitol and vitamin D reduce PTH levels by inhibiting its synthesis and secretion.
Pharmacodynamics:
Paricalcitol reduces iPTH levels and elevates some patients' serum phosphorus and calcium levels. A mathematical model was devised to confirm this relationship. Clinical trials reveal that the drug has low efficacy in patients undergoing hemodialysis or peritoneal dialysis.
Pharmacokinetics:
Absorption:
The absolute bioavailability of Paricalcitol capsules has been observed to be 72 to 86 % in healthy adults, patients with stage 5 chronic kidney disease, or who are on peritoneal dialysis or hemodialysis. The maximum concentration of the drug and area under the curve changed when Paricalcitol was administered to patients with high-fat meals.
Distribution:
Paricalcitol is more than 99.8 % bound to plasma proteins. Paricalcitol’s mean apparent volume of distribution in healthy adults was noted to be 34 liters. In contrast, the drug’s mean apparent volume of distribution after a 4 mcg dose in stage 3 chronic kidney disease patients is between 44 to 46 liters.
Metabolism:
When Paricalcitol 0.48 mcg per kg was administered orally, the parent drug got metabolized, and 2 % of it got eliminated in the feces. Numerous metabolites were detected in urine and feces. In vitro, studies suggest that numerous hepatic and non-hepatic enzymes, including mitochondrial CYP24, CYP3A4, and UGT1A4, metabolize Paricalcitol.
Chemical Taxonomy:
Kingdom - Organic compounds.
Superclass - Lipid and lipid-like molecules.
Class - Steroids and steroid derivatives.
Subclass - Vitamin D and derivatives.
Non-Clinical Toxicology:
Carcinogenesis, Mutagenesis, and Impairment of Fertility:
No clinically significant studies have been conducted on humans to determine Paricalcitol's carcinogenic or mutagenic potential. However, animal studies report an increased incidence of uterine leiomyoma and leiomyosarcoma at certain subcutaneous doses. No alterations in the genetic structure (mutations) associated with Paricalcitol were observed in animal studies. In addition, the drug did not affect fertility when it was administered at a dose 13 times higher than the human dose.
Drug Interactions:
CYP3A Inhibitors - Paricalcitol exposure increases upon concomitant administration with CYP3A inhibitors because they partially metabolize the drug. Hence, it must be administered cautiously with the following CYP3A inhibitors:
-
Boceprevir.
-
Clarithromycin.
-
Conivaptan.
-
Grapefruit juice.
-
Indinavir.
-
Ketoconazole.
-
Itraconazole.
-
Nefazodone.
-
Nelfinadine.
-
Posaconazole.
-
Telaprevir.
Cholestyramine - Cholestyramine are drugs that interrupt the intestinal absorption of fat-soluble vitamins. These drugs can interfere with Paricalcitol absorption. Hence, Paricalcitol capsules must be taken an hour before or four to six hours after cholestyramine.
Mineral Oil - Mineral oils affect Paricalctol’s absorption, so the drug must be taken an hour before consuming these oils.
Overdosage:
Excess administration of Paricalcitol can induce hypercalcemia, hyperphosphatemia, and PTH suppression. General supportive measures, induction of emesis, or gastric lavage can treat Paricalcitol overdosage. However, if the drug passes into the stomach, mineral oil can be used for fecal elimination.
What Are the Adverse Reactions of Paricalcitol?
The adverse reactions of Paricalcitol are listed below:
-
Gastrointestinal Disorders:
-
Abdominal pain.
-
Nausea.
-
Vomiting.
-
Constipation.
-
Diarrhea.
-
-
General Disorders:
-
Vertigo.
-
Chest pain.
-
Edema.
-
Fatigue.
-
-
Immune System Disorders:
-
Hypersensitivity.
-
-
Infections and Infestations:
-
Gastroenteritis.
-
Fungal infections.
-
Sinusitis.
-
Urinary tract infections.
-
Viral infections.
-
Conjunctivitis.
-
Rhinitis.
-
Peritonitis.
-
-
Metabolism and Nutrition Disorders:
-
Hypoglycemia.
-
Fluid overload.
-
Dehydration.
-
-
Musculoskeletal Disorders:
-
Muscle spasm.
-
Arthritis.
-
Back pain.
-
-
Neurological Disorders:
-
Dizziness.
-
Syncope.
-
Headache.
-
Dysgeusia.
-
Depression.
-
-
Respiratory and Thoracic disorders:
-
Cough.
-
Oropharyngeal pain.
-
Asthma.
-
-
Dermatologic Disorders:
-
Pruritus.
-
Rashes.
-
Skin ulcers.
-
-
Renal Disorders:
-
Urinary urgency.
-
-
Vascular Disorders:
-
Hypertension.
-
Hypotension.
-
Use in Specific Populations:
Pregnancy:
Limited data are available on the effects of Paricalcitol in pregnant females. However, mothers with chronic kidney disease must be carefully monitored. Animal studies reported incidences of embryo-fetal loss in pregnant females during organogenesis when the drug was administered at twice the higher dose.
Lactation:
Nothing has been known about the presence of Paricalcitol in breast milk and its effect on the breastfed infant. Animal studies suggest that metabolites of Paricalcitol were found in breast milk. Hence, patients must be advised to avoid breastfeeding during Paricalcitol therapy.
Pediatric Use:
Paricalcitol can be safely administered in children ten to 16 years of age diagnosed with secondary hyperparathyroidism associated with stage 3, 4, or 5 chronic kidney disease. Adequate and well-controlled studies have been done for 12 weeks on 36 pediatric patients to determine their safety and efficacy.
Geriatric Patients:
A clinical trial was done on 220 patients with stage 3 and 4 kidney disease associated with secondary hyperparathyroidism. 49 % of these patients were above 65, whereas 17 % were above 75 years old. However, no clinically significant differences were noted in the overall safety and efficacy of the drug.
Clinical Trial:
Three double-blind, placebo-controlled, and randomized trials were done to evaluate the safety and efficacy of Paricalcitol. The drug was administered to 107 patients, whereas 113 patients received the placebo. Paricalcitol 2 mcg was administered daily, and 4 mcg was given a week thrice. The dose was titrated and increased by 1 mcg every week. More than 30 % reductions were observed in the iPTH levels with Paricalcitol, whereas only 13 % reductions were obtained with placebo.












